Textbook Question
Determine the number of atoms per unit cell for each metal.
(a) Polonium
600
views


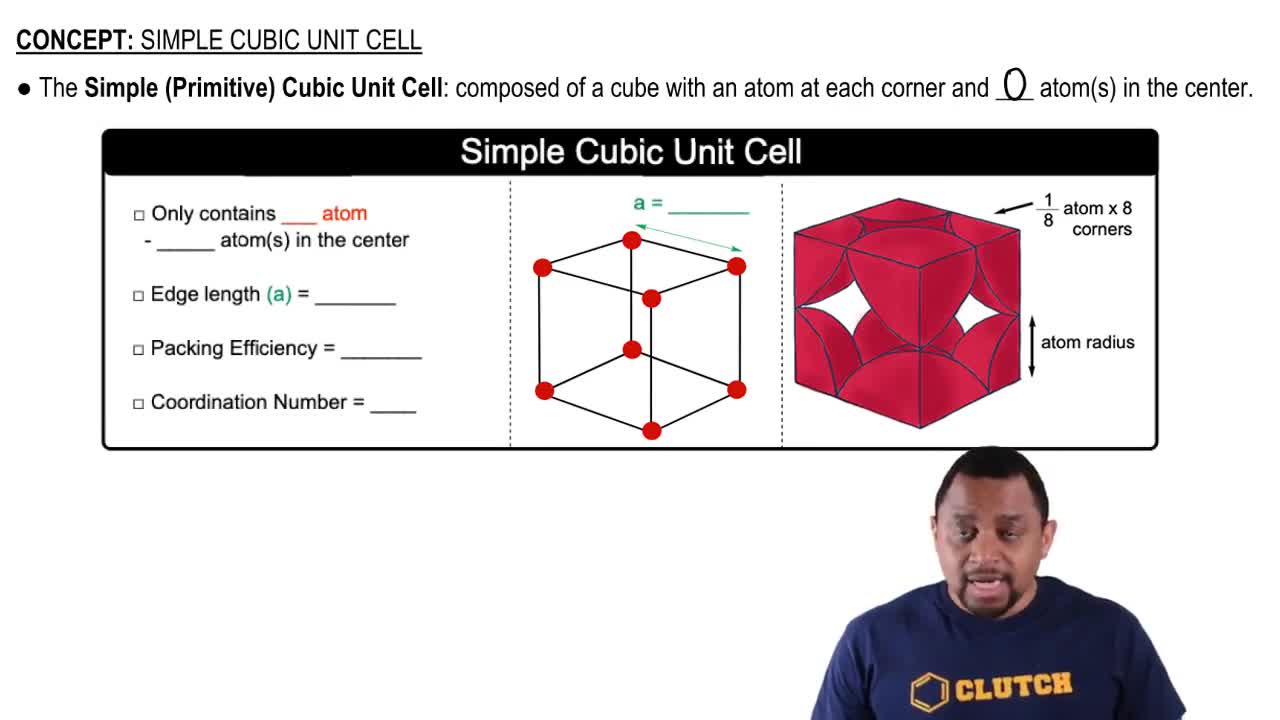

Determine the number of atoms per unit cell for each metal.
(a) Polonium
Determine the number of atoms per unit cell for each metal.
(b) Tungsten
Determine the number of atoms per unit cell for each metal.
(c) Nickel
Molybdenum crystallizes with the body-centered unit cell. The radius of a molybdenum atom is 136 pm. Calculate the edge length of the unit cell and the density of molybdenum
Rhodium has a density of 12.41 g/cm3 and crystallizes with the face-centered cubic unit cell. Calculate the radius of a rhodium atom.
Barium has a density of 3.59 g/cm3 and crystallizes with the body-centered cubic unit cell. Calculate the radius of a barium atom.