Here are the essential concepts you must grasp in order to answer the question correctly.
Significant Figures
Significant figures are the digits in a number that contribute to its precision. This includes all non-zero digits, any zeros between significant digits, and trailing zeros in the decimal portion. Understanding significant figures is crucial for accurately reporting measurements and calculations in chemistry, as it reflects the precision of the data used.
Recommended video:
Significant Figures Example
Order of Operations
The order of operations is a set of rules that dictates the sequence in which calculations should be performed to ensure accurate results. In chemistry and mathematics, the acronym PEMDAS (Parentheses, Exponents, Multiplication and Division, Addition and Subtraction) is commonly used. Following this order is essential when performing calculations involving multiple operations, such as the one in the question.
Recommended video:
Scientific Notation Mixed Operations
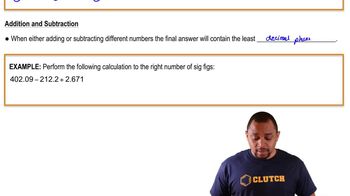
Subtraction and Significant Figures
When performing subtraction, the result should be reported with the same number of decimal places as the measurement with the least number of decimal places. This rule ensures that the precision of the result reflects the least precise measurement involved in the calculation. In the given question, this principle will guide how to express the final answer after performing the subtraction.
Recommended video:
Significant Figures in Addition and Substraction
 Verified step by step guidance
Verified step by step guidance