Textbook Question
Write the Lewis structure for each molecule (octet rule not followed). a. BBr3 b. NO c. ClO2
829
views
2
comments

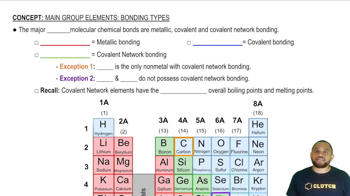


Write the Lewis structure for each molecule (octet rule not followed). a. BBr3 b. NO c. ClO2
Write Lewis structures for each molecule or ion. Use expanded octets as necessary. a. PF5
Write Lewis structures for each molecule or ion. Use expanded octets as necessary. b. AsF6-
Order these compounds in order of decreasing carbon–carbon bond length: HCCH, H2CCH2, H3CCH3.
Which of the two compounds, H2NNH2 and HNNH, has the strongest nitrogen-nitrogen bond, and which has the shorter nitrogen-nitrogen bond.
Hydrogenation reactions are used to add hydrogen across double bonds in hydrocarbons and other organic compounds. Use average bond energies to calculate ΔHrxn for the hydrogenation reaction. H2C=CH2(g) + H2(g) → H3C–CH3(g)