Arrange these elements in order of increasing metallic character: Fr, Sb, In, S, Ba, Se.
Life on Earth evolved based on the element carbon. Based on periodic properties, what two or three elements would you expect to be most like carbon?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
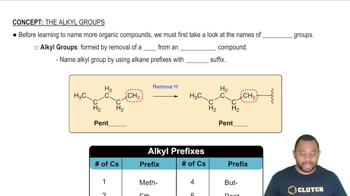
Periodic Trends

Group Similarities
Covalent Bonding
Arrange these elements in order of decreasing metallic character: Sr, N, Si, P, Ga, Al.
Both vanadium and its 3+ ion are paramagnetic. Refer to their electron configurations to explain this statement.
The elements with atomic numbers 35 and 53 have similar chemical properties. Based on their electronic configurations, predict the atomic number of a heavier element that also should share these chemical properties.
You have cracked a secret code that uses elemental symbols to spell words. The code uses numbers to designate the elemental symbols. Each number is the sum of the atomic number and the highest principal quantum number of the highest occupied orbital of the element whose symbol is to be used. The message may be written forward or backward. Decode the following messages: a. 10, 12, 58, 11, 7, 44, 63, 66
Use Coulomb's law to calculate the ionization energy in kJ>mol of an atom composed of a proton and an electron separated by 100.00 pm. What wavelength of light has sufficient energy to ionize the atom?
