A kilogram of aluminum metal and a kilogram of water are each warmed to 75 °C and placed in two identical insulated containers. One hour later, the two containers are opened and the temperature of each substance is measured. The aluminum has cooled to 35 °C, while the water has cooled only to 66 °C. Explain this difference.
Suppose that 25 g of each substance is initially at 27.0 °C. What is the final temperature of each substance upon absorbing 2.35 kJ of heat? c. aluminum
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Specific Heat Capacity

Heat Transfer

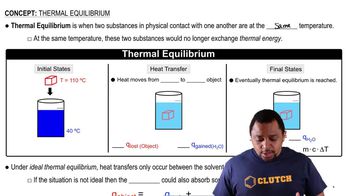
Thermal Equilibrium
How much heat is required to warm 1.50 L of water from 25.0 °C to 100.0 °C? (Assume a density of 1.0 g/mL for the water.)
How much heat is required to warm 1.50 kg of sand from 25.0 °C to 100.0 °C?
An unknown mass of each substance, initially at 23.0 °C, absorbs 1.95 × 103 J of heat. The final temperature is recorded. Find the mass of each substance.
a. Pyrex glass (Tf = 55.4°C)
b. sand (Tf = 62.1°C)
c. ethanol (Tf = 44.2°C)
d. water (Tf = 32.4°C)
How much work (in J) is required to expand the volume of a pump from 0.0 L to 2.5 L against an external pressure of 1.1 atm?
The average human lung expands by about 0.50 L during each breath. If this expansion occurs against an external pressure of 1.0 atm, how much work (in J) is done during the expansion?
