Complete and balance each equation. If no reaction occurs, write 'NO REACTION.' d. NH4Cl(aq) + AgNO3(aq) →
Write a molecular equation for the precipitation reaction that occurs (if any) when each pair of aqueous solutions is mixed. If no reaction occurs, write 'NO REACTION.' a. sodium chloride and lead(II) acetate b. potassium sulfate and strontium iodide c. cesium chloride and calcium sulfide d. chromium(III) nitrate and sodium phosphate
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Precipitation Reactions

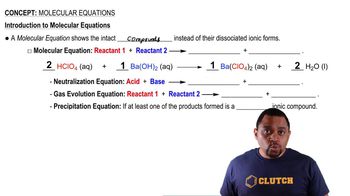
Molecular Equations
Solubility Rules

Write a molecular equation for the precipitation reaction that occurs (if any) when each pair of aqueous solutions is mixed. If no reaction occurs, write 'NO REACTION.' a. potassium carbonate and lead(II) nitrate
Write a molecular equation for the precipitation reaction that occurs (if any) when each pair of aqueous solutions is mixed. If no reaction occurs, write 'NO REACTION.' c. copper(II) nitrate and magnesium sulfide
Write balanced complete ionic and net ionic equations for each reaction. a. HCl(aq) + LiOH(aq) → H2O(l) + LiCl(aq)
Write balanced complete ionic and net ionic equations for each reaction. CaS(aq) + CuCl2(aq) → CuS(s) + CaCl2(aq)
Write balanced complete ionic and net ionic equations for each reaction. d. Na3PO4(aq) + NiCl2(aq) → Ni3(PO4)2(s) + NaCl(aq)
