Textbook Question
Write the name from the formula or the formula from the name for each hydrated ionic compound.
a. cobalt(II) phosphate octahydrate
b. BeCl2•2 H2O
c. chromium(III) phosphate trihydrate
d. LiNO2•H2O
617
views
 Verified step by step guidance
Verified step by step guidance


Write the name from the formula or the formula from the name for each hydrated ionic compound.
a. cobalt(II) phosphate octahydrate
b. BeCl2•2 H2O
c. chromium(III) phosphate trihydrate
d. LiNO2•H2O
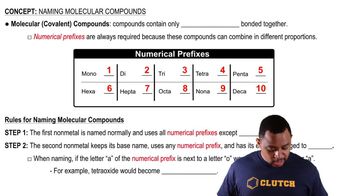
Name each molecular compound. a. CO b. NI3 c. SiCl4 d. N4Se4
Name each molecular compound. a. SO3 c. BrF5
Name each molecular compound. d. NO
Write the formula for each molecular compound. a. phosphorus trichloride b. chlorine monoxide c. disulfur tetrafluoride
Write the formula for each molecular compound. d. phosphorus pentafluoride