How many molecules of ethanol (C2H5OH) (the alcohol in alcoholic beverages) are present in 145 mL of ethanol? The density of ethanol is 0.789 g/cm3.
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. c. sulfurous acid

Verified Solution
Key Concepts
Chemical Formula
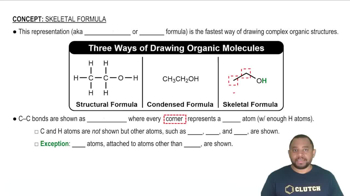
Mass Percent Composition

Molar Mass

A drop of water has a volume of approximately 0.05 mL. How many water molecules does it contain? The density of water is 1.0 g/cm3.
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. a. potassium chromate b. lead(II) phosphate
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. d. cobalt(II) bromide
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. c. nitrogen triiodide
A Freon leak in the air-conditioning system of an old car releases 25 g of CF2Cl2 per month. What mass of chlorine does this car emit into the atmosphere each year?
