Determine whether or not the mixing of each pair of solutions results in a buffer. a. 75.0 mL of 0.10 M HF; 55.0 mL of 0.15 M NaF b. 150.0 mL of 0.10 M HF; 135.0 mL of 0.175 M HCl d. 125.0 mL of 0.15 M CH3NH2; 120.0 mL of 0.25 M CH3NH3Cl
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 M in HCO3- and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1).
c. Given the volume from part (b), what mass of NaOH can be neutralized before the pH rises above 7.8?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
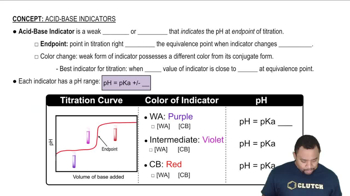
Key Concepts
Buffer Systems

pKa and Acid-Base Equilibrium
Neutralization Reactions

Determine whether or not the mixing of each pair of solutions results in a buffer. c. 165.0 mL of 0.10 M HF; 135.0 mL of 0.050 M KOH
Blood is buffered by carbonic acid and the bicarbonate ion. Normal blood plasma is 0.024 M in HCO3- and 0.0012 M H2CO3 (pKa1 for H2CO3 at body temperature is 6.1).
a. What is the pH of blood plasma?
The fluids within cells are buffered by H2PO4- and HPO42- . b. Could a buffer system employing H3PO4 as the weak acid and H2PO4- as the weak base be used as a buffer system within cells? Explain.
Which buffer system is the best choice to create a buffer with pH = 9.00? For the best system, calculate the ratio of the masses of the buffer components required to make the buffer. HF/KF HNO2/KNO2 NH3/NH4Cl HClO/KClO
A 500.0-mL buffer solution is 0.100 M in HNO2 and 0.150 M in KNO2. Determine if each addition would exceed the capacity of the buffer to neutralize it. a. 250 mg NaOH
