Textbook Question
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). b. HCHO2
512
views
 Verified step by step guidance
Verified step by step guidance
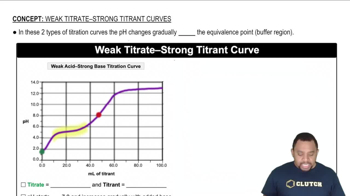


Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). b. HCHO2
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). c. H2SO4
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (Ka). d. H2CO3
Pick the stronger base from each pair. c. F– or ClO–
Calculate [H3O+] and [OH–] for each solution at 25 °C. a. pH = 8.55
Calculate [H3O+] and [OH–] for each solution at 25 °C. b. pH = 11.23 c. pH = 2.87