Textbook Question
Determine the [H3O+] and pH of a 0.100 M solution of benzoic acid.
801
views
 Verified step by step guidance
Verified step by step guidance


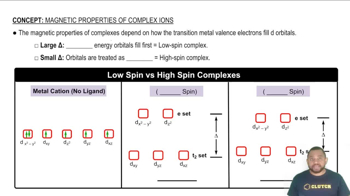
Determine the [H3O+] and pH of a 0.100 M solution of benzoic acid.
Determine the [H3O+] and pH of a 0.200 M solution of formic acid.
Determine the pH of an HNO2 solution of each concentration. In which cases can you not make the simplifying assumption that x is small? a. 0.500 M b. 0.100 M c. 0.0100 M
A 0.185 M solution of a weak acid (HA) has a pH of 2.95. Calculate the acid ionization constant (Ka) for the acid.
Determine the percent ionization of a 0.125 M HCN solution.
Determine the percent ionization of a 0.225 M solution of benzoic acid.