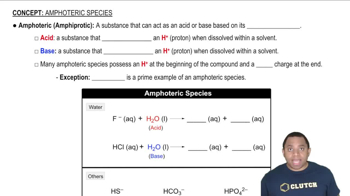
Write an expression for the equilibrium constant of each chemical equation.
a. SbCl5(g) ⇌ SbCl3(g) + Cl2(g)
b.2 BrNO(g) ⇌ 2 NO(g) + Br2(g)
c. CH4(g) + 2 H2S(g) ⇌ CS2(g) + 4 H2(g)
d. 2 CO(g) + O2(g) ⇌ 2 CO2(g)



Write an expression for the equilibrium constant of each chemical equation.
a. SbCl5(g) ⇌ SbCl3(g) + Cl2(g)
b.2 BrNO(g) ⇌ 2 NO(g) + Br2(g)
c. CH4(g) + 2 H2S(g) ⇌ CS2(g) + 4 H2(g)
d. 2 CO(g) + O2(g) ⇌ 2 CO2(g)
Find and fix each mistake in the equilibrium constant expressions. a. 2 H2S(g) ⇌ 2 H2(g) + S2(g) K = [H2][S2]/[H2S]
When this reaction comes to equilibrium, will the concentrations of the reactants or products be greater? Does the answer to this question depend on the initial concentrations of the reactants and products? A(g)+B(g) ⇌ 2 C(g) Kc = 1.4⨉10-5
Ethene (C2H4) can be halogenated by this reaction: C2H4(g) + X2(g) ⇌ C2H4X2(g) where X2 can be Cl2 (green), Br2 (brown), or I2 (purple). Examine the three figures representing equilibrium concentrations in this reaction at the same temperature for the three different hal- ogens. Rank the equilibrium constants for the three reactions from largest to smallest.
H2 and I2 are combined in a flask and allowed to react according to the reaction: H2(g) + I2(g) ⇌ 2 HI(g) Examine the figures (sequential in time) and answer the questions: a. Which figure represents the point at which equilibrium is reached?