Consider this energy diagram:
d. Is the overall reaction endothermic or exothermic?
 Verified step by step guidance
Verified step by step guidance


Consider this energy diagram:
d. Is the overall reaction endothermic or exothermic?
Consider the reaction in which HCl adds across the double bond of ethene: HCl + H2C=CH2 → H3C-CH2Cl The following mechanism, with the accompanying energy diagram, has been suggested for this reaction:
Step 1 HCl + H2C=CH2 → H3C=CH2+ + Cl-
Step 2 H3C=CH2+ + Cl- → H3C-CH2Cl
a. Based on the energy diagram, determine which step is rate limiting.
Consider the reaction in which HCl adds across the double bond of ethene: HCl + H2C=CH2 → H3C-CH2Cl The following mechanism, with the accompanying energy diagram, has been suggested for this reaction:
Step 1 HCl + H2C=CH2 → H3C=CH2+ + Cl-
Step 2 H3C=CH2+ + Cl- → H3C-CH2Cl
b. What is the expected order of the reaction based on the proposed mechanism?
The desorption (leaving of the surface) of a single molecular layer of n-butane from a single crystal of aluminum oxide is found to be first order with a rate constant of 0.128/s at 150 K. b. If the surface is initially completely covered with n-butane at 150 K, how long will it take for 25% of the molecules to desorb (leave the surface)? For 50% to desorb?
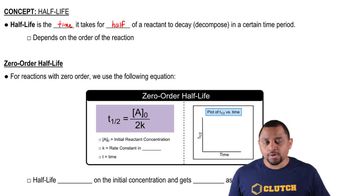
The evaporation of a 120-nm film of n-pentane from a single crystal of aluminum oxide is zero order with a rate constant of 1.92⨉1013 molecules/cm2•s at 120 K. a. If the initial surface coverage is 8.9⨉1016 molecules/cm2, how long will it take for one-half of the film to evaporate?
The kinetics of this reaction were studied as a function of temperature. (The reaction is first order in each reactant and second order overall.)
C2H5Br(aq) + OH- (aq) → C2H5OH(l) + Br- (aq)
Temperature (°C) k (L,mol •s)
25 8.81⨉10-5
35 0.000285
45 0.000854
55 0.00239
65 0.00633
b. Determine the rate constant at 15 °C.