The half-life for the radioactive decay of C-14 is 5730 years and is independent of the initial concentration. How long does it take for 25% of the C-14 atoms in a sample of C-14 to decay?
The activation energy of a reaction is 56.8 kJ/mol and the frequency factor is 1.5⨉1011/ s. Calculate the rate constant of the reaction at 25 °C.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Activation Energy

Arrhenius Equation
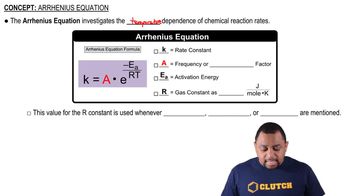
Rate Constant

The half-life for the radioactive decay of C-14 is 5730 years and is independent of the initial concentration. If a sample of C-14 initially contains 1.5 mmol of C-14, how many millimoles are left after 2255 years?
The diagram shows the energy of a reaction as the reaction progresses. Label each blank box in the diagram.
a. reactants b. products c. activation energy (Ea) d. enthalpy of reaction (ΔHrxn)
The rate constant (k) for a reaction was measured as a function of temperature. A plot of ln k versus 1/T (in K) is linear and has a slope of -7445 K. Calculate the activation energy for the reaction.
The data shown here were collected for the first-order reaction: N2O(g) → N2(g) + O(g) Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.
Temperature (K) Rate Constant (1 , s)
800 3.24⨉10- 5
900 0.00214
1000 0.0614
1100 0.955
The tabulated data show the rate constant of a reaction measured at several different temperatures. Use an Arrhenius plot to determine the activation barrier and frequency factor for the reaction.
Temperature (K) Rate Constant (1 , s)
300 0.0134
310 0.0407
320 0.114
330 0.303
340 0.757
