Which of these two reactions would you expect to have the smaller orientation factor? Explain. a. O(g) + N2(g) → NO( g) + N(g) b. NO(g) + Cl2(g) → NOCl( g) + Cl(g)
Consider this three-step mechanism for a reaction:
Cl2 (g) k1⇌k2 2 Cl (g) Fast
Cl (g) + CHCl3 (g) →k3 HCl (g) + CCl3 (g) Slow
Cl (g) + CCl3 (g) →k4 CCl4 (g) Fast
c. What is the predicted rate law?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
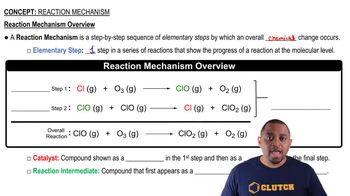
Reaction Mechanism
Rate Law

Elementary Steps

Consider this overall reaction, which is experimentally observed to be second order in AB and zero order in C: AB + C → A + BC Is the following mechanism valid for this reaction? AB + AB →k1 AB2 + A Slow AB2 + C → k2 AB + BC Fast
Consider this three-step mechanism for a reaction:
Cl2 (g) k1⇌k2 2 Cl (g) Fast
Cl (g) + CHCl3 (g) →k3 HCl (g) + CCl3 (g) Slow
Cl (g) + CCl3 (g) →k4 CCl4 (g) Fast
a. What is the overall reaction?
Consider this two-step mechanism for a reaction: NO2(g) + Cl2(g) → k1 ClNO2(g) + Cl g) Slow NO2(g) + Cl(g) →k2 ClNO2(g) Fast b. Identify the intermediates in the mechanism.
Many heterogeneous catalysts are deposited on high-surfacearea supports. Why?
Suppose that the reaction A¡products is exothermic and has an activation barrier of 75 kJ/mol. Sketch an energy diagram showing the energy of the reaction as a function of the progress of the reaction. Draw a second energy curve showing the effect of a catalyst.
