Which solid in each pair has the higher melting point and why?
a. TiO2(s) or HOOH(s)
b. CCl4(s) or SiCl4(s)
c. Kr(s) or Xe(s)

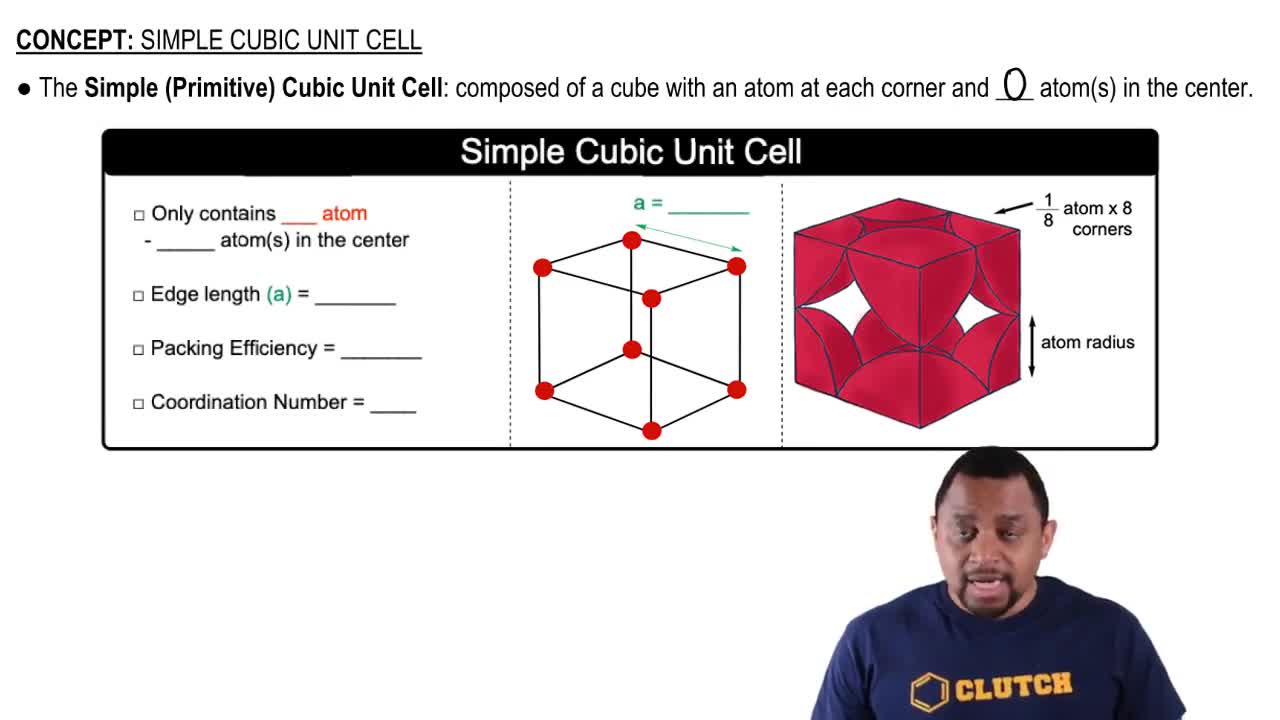

Which solid in each pair has the higher melting point and why?
a. TiO2(s) or HOOH(s)
b. CCl4(s) or SiCl4(s)
c. Kr(s) or Xe(s)
Which solid in each pair has the higher melting point and why? d. NaCl(s) or CaO(s)
Which solid in each pair has the higher melting point and why?
a. Fe(s) or CCl4(s)
b. KCl(s) or HCl(s)
c. Ti(s) or Ne(s)
d. H2O(s) or H2S(s)
Identify the structure of each of the two unit cells shown in Problem 48 as the rock salt structure, zinc blende structure, fluorite structure, antifluorite structure, or none of these.
Classify each of the following as a component of a silicate ceramic, an oxide ceramic, or a nonoxide ceramic. a. B4C
Classify each of the following as a component of a silicate ceramic, an oxide ceramic, or a nonoxide ceramic. b. Mg2SiO4