Draw an energy diagram for HCl. Predict the bond order and make a sketch of the lowest energy bonding molecular orbital.
 Tro 5th Edition
Tro 5th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 88b
Problem 88bThe structure of acetylsalicylic acid (aspirin) is shown here. How many sigma bonds?
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Sigma Bonds
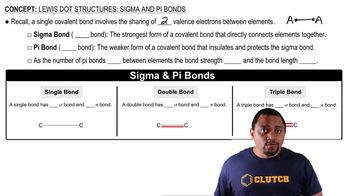
Molecular Structure

Counting Bonds in Organic Compounds

The genetic code is based on four different bases with the structures shown here. Assign a geometry and hybridization to each interior atom in these four bases. d. guanine
The structure of acetylsalicylic acid (aspirin) is shown here. How many π bonds are present in acetylsalicylic acid?
Most vitamins can be classified as either fat soluble, which results in their tendency to accumulate in the body (so that taking too much can be harmful), or water soluble, which results in their tendency to be quickly eliminated from the body in urine. Examine the structural formulas and space-filling models of these vitamins and determine whether each one is fat soluble (mostly nonpolar) or water soluble (mostly polar). (a) vitamin C
Most vitamins can be classified as either fat soluble, which results in their tendency to accumulate in the body (so that taking too much can be harmful), or water soluble, which results in their tendency to be quickly eliminated from the body in urine. Examine the structural formulas and space-filling models of these vitamins and determine whether each one is fat soluble (mostly nonpolar) or water soluble (mostly polar). (c) niacin (vitamin B3)
Water does not easily remove grease from dishes or hands because grease is nonpolar and water is polar. The addition of soap to water, however, allows the grease to dissolve. Study the structure of sodium stearate (a soap) and describe how it works.