Determine the electron geometry, molecular geometry, and idealized bond angles for each molecule. In which cases do you expect deviations from the idealized bond angle? a. CF4 b. NF3 c. OF2 d. H2S
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
All textbooks Tro 5th Edition
Tro 5th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 39c
Problem 39c
 Tro 5th Edition
Tro 5th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 39c
Problem 39cChapter 11, Problem 39c
Determine the molecular geometry and sketch each molecule or ion using the bond conventions shown in 'Representing Molecular Geometries on Paper' in Section 10.4. c. IF2-
 Verified step by step guidance
Verified step by step guidance1
Determine the total number of valence electrons for IF_2^-. Iodine (I) has 7 valence electrons, each fluorine (F) has 7 valence electrons, and the negative charge adds 1 more electron.
Calculate the total number of valence electrons: 7 (I) + 2*7 (F) + 1 (negative charge) = 22 valence electrons.
Draw the Lewis structure: Place iodine (I) in the center, and arrange the two fluorine (F) atoms around it. Distribute the electrons to satisfy the octet rule for each atom.
Determine the electron pair geometry using VSEPR theory. Count the number of bonding pairs and lone pairs around the central iodine atom.
Identify the molecular geometry based on the arrangement of bonding pairs and lone pairs around the central atom. Sketch the molecule using the bond conventions, showing the lone pairs and bond angles.

Verified Solution
Video duration:
1mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
VSEPR Theory
Valence Shell Electron Pair Repulsion (VSEPR) Theory is a model used to predict the geometry of individual molecules based on the repulsion between electron pairs in the valence shell of the central atom. According to VSEPR, electron pairs will arrange themselves as far apart as possible to minimize repulsion, leading to specific molecular shapes.
Recommended video:
Guided course
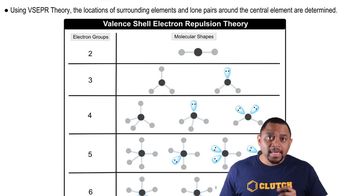
Molecular Shapes and VSEPR
Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms within a molecule. It is determined by the number of bonding pairs and lone pairs of electrons around the central atom, which influences the overall shape, such as linear, trigonal planar, tetrahedral, or octahedral.
Recommended video:
Guided course

Molecular Geometry with Two Electron Groups
Lewis Structures
Lewis structures are diagrams that represent the bonding between atoms in a molecule and the lone pairs of electrons that may exist. They are essential for visualizing the arrangement of electrons and help in predicting molecular geometry by showing how atoms are connected and the distribution of electron pairs.
Recommended video:
Guided course

Lewis Dot Structures: Ions
Related Practice
Textbook Question
1172
views
Textbook Question
Which species has the smaller bond angle, H3O+ or H2O? Explain.
1068
views
Textbook Question
Which species has the smaller bond angle, ClO4- or ClO3- ? Explain.
998
views
Textbook Question
Determine the molecular geometry and sketch each molecule or ion using the bond conventions shown in 'Representing Molecular Geometries on Paper' in Section 10.4. d. IBr4-
778
views
Textbook Question
Determine the molecular geometry and sketch each molecule or ion, using the bond conventions shown in 'Representing Molecular Geometries on Paper' in Section 10.4. b. SCl6
543
views
Textbook Question
Determine the molecular geometry and sketch each molecule or ion, using the bond conventions shown in 'Representing Molecular Geometries on Paper' in Section 10.4. c. PF5
456
views