Determine the molecular geometry about each interior atom and draw each molecule. (Skeletal structure is indicated in parentheses.)
a. C2H2 (skeletal structure HCCH)
b. C2H4 (skeletal structure H2CCH2)
c. C2H6 (skeletal structure H3CCH3)
 Tro 5th Edition
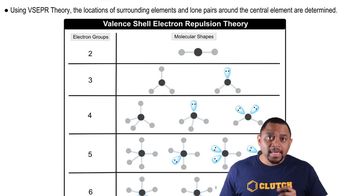
Tro 5th Edition Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory
Ch.11 - Chemical Bonding II: Molecular Shapes, VSEPR & MO Theory Problem 42c
Problem 42cDetermine the molecular geometry about each interior atom and sketch each molecule. c. N2H4 (skeletal structure H2NNH2)
 Verified step by step guidance
Verified step by step guidance


Determine the molecular geometry about each interior atom and draw each molecule. (Skeletal structure is indicated in parentheses.)
a. C2H2 (skeletal structure HCCH)
b. C2H4 (skeletal structure H2CCH2)
c. C2H6 (skeletal structure H3CCH3)
Determine the molecular geometry about each interior atom and sketch each molecule. a. N2
Determine the molecular geometry about each interior atom and sketch each molecule. b. N2H2 (skeletal structure HNNH)
Each ball-and-stick model shows the electron and molecular geometry of a generic molecule. Explain what is wrong with each molecular geometry and provide the correct molecular geometry, given the number of lone pairs and bonding groups on the central atom. (c)
Determine the geometry about each interior atom in each molecule and sketch the molecule. (Skeletal structure is indicated in parentheses.) a. CH3OH (H3COH) b. CH3OCH3 (H3COCH3)
Determine the geometry about each interior atom in each molecule and sketch the molecule. (Skeletal structure is indicated in parentheses.) c. H2O2 (HOOH)