Here are the essential concepts you must grasp in order to answer the question correctly.
Electron Configuration
Electron configuration describes the distribution of electrons in an atom's orbitals. For vanadium (V), with an atomic number of 23, the electron configuration is [Ar] 3d³ 4s². Understanding this configuration is crucial to determine how many unpaired electrons are present, which directly relates to the paramagnetic property.
Recommended video:
Electron Configuration Example
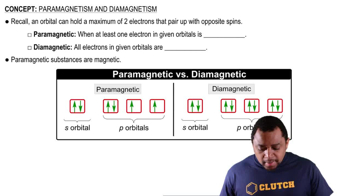
Paramagnetism
Paramagnetism is a property of materials that are attracted to magnetic fields due to the presence of unpaired electrons. Both vanadium and its 3+ ion (V³⁺) have unpaired electrons in their electron configurations, which allows them to exhibit paramagnetic behavior. The presence of unpaired electrons is essential for this magnetic property.
Recommended video:
Paramagnetism and Diamagnetism
Ionization and Electron Loss
Ionization involves the removal of electrons from an atom, resulting in the formation of ions. For vanadium, when it loses three electrons to form V³⁺, the electron configuration changes to [Ar] 3d². Analyzing how the loss of electrons affects the number of unpaired electrons helps explain why both vanadium and V³⁺ remain paramagnetic.
Recommended video:
 Verified step by step guidance
Verified step by step guidance