A kilogram of aluminum metal and a kilogram of water are each warmed to 75 °C and placed in two identical insulated containers. One hour later, the two containers are opened and the temperature of each substance is measured. The aluminum has cooled to 35 °C, while the water has cooled only to 66 °C. Explain this difference.
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Specific Heat Capacity

Thermal Equilibrium
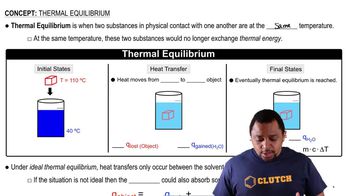
Insulation and Heat Loss

The air in an inflated balloon (defined as the system) warms over a toaster and absorbs 115 J of heat. As it expands, it does 77 kJ of work. What is the change in internal energy for the system?
We pack two identical coolers for a picnic, placing 24 12-ounce soft drinks and five pounds of ice in each. However, the drinks that we put into cooler A were refrigerated for several hours before they were packed in the cooler, while the drinks that we put into cooler B were at room temperature. When we open the two coolers three hours later, most of the ice in cooler A is still present, while nearly all of the ice in cooler B has melted. Explain this difference.
How much heat is required to warm 1.50 L of water from 25.0 °C to 100.0 °C? (Assume a density of 1.0 g/mL for the water.)
How much heat is required to warm 1.50 kg of sand from 25.0 °C to 100.0 °C?
Suppose that 25 g of each substance is initially at 27.0 °C. What is the final temperature of each substance upon absorbing 2.35 kJ of heat? c. aluminum
