Many home barbeques are fueled with propane gas (C3H8). What mass of carbon dioxide (in kg) is produced upon the complete combustion of 18.9 L of propane (approximate contents of one 5-gallon tank)? Assume that the density of the liquid propane in the tank is 0.621 g/mL. (Hint: Begin by writing a balanced equation for the combustion reaction.)
 Verified step by step guidance
Verified step by step guidance
Verified Solution
Key Concepts
Combustion Reaction

Stoichiometry

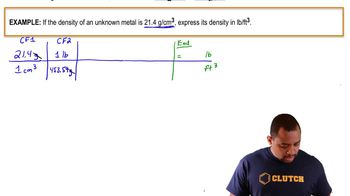
Density and Volume Conversion
The combustion of gasoline produces carbon dioxide and water. Assume gasoline to be pure octane (C8H18) and calculate the mass (in kg) of carbon dioxide that is added to the atmosphere per 1.0 kg of octane burned. (Hint: Begin by writing a balanced equation for the combustion reaction.)
Aspirin can be made in the laboratory by reacting acetic anhydride (C4H6O3) with salicylic acid (C7H6O3) to form aspirin (C9H8O4) and acetic acid (C2H4O2). The balanced equation is: C4H6O3 + C7H6O3 → C9H8O4 + C2H4O2 In a laboratory synthesis, a student begins with 3.00 mL of acetic anhydride (density = 1.08 g/mL) and 1.25 g of salicylic acid. Once the reaction is complete, the student collects 1.22 g of aspirin. Determine the limiting reactant. Determine the theoretical yield of aspirin. Determine the percent yield for the reaction.
The combustion of liquid ethanol (C2H5OH) produces carbon dioxide and water. After 4.62 mL of ethanol (density = 0.789 g/mL) is allowed to burn in the presence of 15.55 g of oxygen gas, 3.72 mL of water (density = 1.00 g/mL) is collected. Determine the percent yield for the reaction. (Hint: Write a balanced equation for the combustion of ethanol.)
A hydrochloric acid solution will neutralize a sodium hydroxide solution. Look at the molecular views showing one beaker of HCl and four beakers of NaOH. Which NaOH beaker will just neutralize the HCl beaker? Begin by writing a balanced chemical equation for the neutralization reaction.
