Here are the essential concepts you must grasp in order to answer the question correctly.
Molecular Compounds
Molecular compounds are formed when two or more nonmetals bond together by sharing electrons. They are characterized by covalent bonds, which involve the sharing of electron pairs between atoms. The properties of molecular compounds, such as their state at room temperature and solubility, differ significantly from ionic compounds, which are formed from metal and nonmetal ions.
Recommended video:
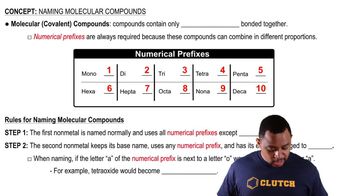
Naming Molecular Compounds
Naming Conventions
The naming of molecular compounds follows specific conventions, often using prefixes to indicate the number of atoms of each element present in the compound. For example, 'mono-' indicates one, 'di-' indicates two, and so forth. In the case of chlorine monoxide, the prefix 'mono-' is used for the single oxygen atom, while chlorine retains its elemental name.
Recommended video:
Chemical Formulas
A chemical formula represents the composition of a compound using symbols for the elements and numerical subscripts to indicate the number of atoms of each element. For molecular compounds, the formula reflects the actual number of atoms in a molecule. In the case of chlorine monoxide, the formula is ClO, indicating one chlorine atom and one oxygen atom.
Recommended video: