Textbook Question
Refer to the nomenclature flowchart (Figure 3.11) to name each compound. a. KClO3 b. I2O5 c. PbSO4
497
views

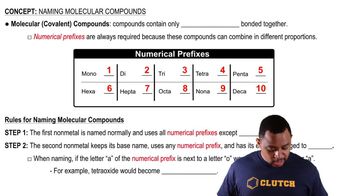


Refer to the nomenclature flowchart (Figure 3.11) to name each compound. a. KClO3 b. I2O5 c. PbSO4
Calculate the mass percent composition of nitrogen in each nitrogen-containing compound. a. N2O b. NO
Write the formula for each ionic compound. b. copper(I) iodate c. lead(II) chromate e. potassium hydroxide
Name each ionic compound. a. SnCl4 b. PbI2 c. Fe2O3 d. CuI2 e. HgBr2 f. CrCl2
How many molecules are in each sample? b. 389 g CBr4
The elemental mass percent composition of ibuprofen (a nonsteroidal anti-inflammatory drug [NSAID]) is 75.69% C, 8.80% H, and 15.51% O. Determine the empirical formula of ibuprofen.