A drop of water has a volume of approximately 0.05 mL. How many water molecules does it contain? The density of water is 1.0 g/cm3.
Ch.3 - Molecules, Compounds & Chemical Equations
Chapter 3, Problem 123d
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. d. cobalt(II) bromide
 Verified step by step guidance
Verified step by step guidance1
Identify the chemical formula of cobalt(II) bromide. Cobalt(II) indicates a +2 charge on cobalt, and bromide has a -1 charge. Therefore, the formula is CoBr₂.
Calculate the molar mass of cobalt(II) bromide. Use the periodic table to find the atomic masses: cobalt (Co) is approximately 58.93 g/mol and bromine (Br) is approximately 79.90 g/mol.
Determine the total molar mass of CoBr₂ by adding the atomic mass of cobalt and twice the atomic mass of bromine: \(58.93 + 2 \times 79.90\).
Calculate the mass percent of cobalt in CoBr₂. Divide the atomic mass of cobalt by the total molar mass of CoBr₂ and multiply by 100 to get the percentage.
Calculate the mass percent of bromine in CoBr₂. Divide the total mass of bromine (2 \times 79.90) by the total molar mass of CoBr₂ and multiply by 100 to get the percentage.

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Formula
A chemical formula represents the composition of a compound, indicating the types and numbers of atoms present. For cobalt(II) bromide, the formula is CoBr2, which shows that each formula unit contains one cobalt atom and two bromine atoms. Understanding how to derive the formula from the compound's name is essential for further calculations.
Recommended video:
Guided course
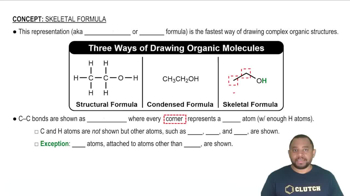
Skeletal Formula
Mass Percent Composition
Mass percent composition is a way to express the relative amounts of each element in a compound as a percentage of the total mass. It is calculated by dividing the mass of each element in one mole of the compound by the molar mass of the compound, then multiplying by 100. This concept is crucial for determining how much of each element contributes to the overall mass of the compound.
Recommended video:
Guided course

Mass Percent Calculation
Molar Mass
Molar mass is the mass of one mole of a substance, typically expressed in grams per mole (g/mol). It is calculated by summing the atomic masses of all atoms in the chemical formula. For cobalt(II) bromide, the molar mass is essential for calculating the mass percent composition, as it provides the total mass needed for the percentage calculations.
Recommended video:
Guided course

Molar Mass Concept
Related Practice
Textbook Question
7694
views
Textbook Question
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. a. potassium chromate b. lead(II) phosphate
670
views
Textbook Question
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. c. sulfurous acid
500
views
Textbook Question
Determine the chemical formula of each compound and then use it to calculate the mass percent composition of each constituent element. c. nitrogen triiodide
569
views
Textbook Question
A Freon leak in the air-conditioning system of an old car releases 25 g of CF2Cl2 per month. What mass of chlorine does this car emit into the atmosphere each year?
3307
views
Open Question
A Freon leak in the air-conditioning system of a large building releases 12 kg of CHF2Cl per month. If the leak is allowed to continue, how many kilograms of Cl will be emitted into the atmosphere each year?
