Here are the essential concepts you must grasp in order to answer the question correctly.
Hydrocarbons
Hydrocarbons are organic compounds composed solely of hydrogen and carbon atoms. They serve as the fundamental building blocks of organic chemistry and can be classified into different categories based on their bonding and structure. The main types include alkanes, alkenes, and alkynes, each exhibiting distinct properties and reactivity.
Recommended video:
Alkanes
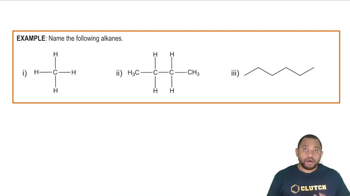
Alkanes are saturated hydrocarbons characterized by single bonds between carbon atoms, following the general formula CnH2n+2. They are typically less reactive than alkenes and alkynes due to the absence of double or triple bonds. The example provided, H3C-CH2-CH2-CH3, is a straight-chain alkane known as butane.
Recommended video:
Alkenes and Alkynes
Alkenes are unsaturated hydrocarbons containing at least one carbon-carbon double bond, represented by the general formula CnH2n. Alkynes, on the other hand, have at least one carbon-carbon triple bond, following the formula CnH2n-2. These structural differences lead to varying chemical properties and reactivity, making them important in various chemical reactions.
Recommended video:
 Verified step by step guidance
Verified step by step guidance