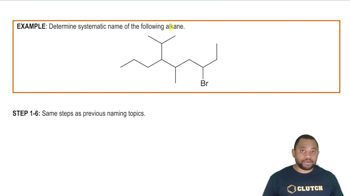
Here are the essential concepts you must grasp in order to answer the question correctly.
Disubstituted Benzene
Disubstituted benzene refers to a benzene ring that has two substituent groups attached to it. The positions of these substituents can significantly affect the chemical properties and reactivity of the compound. The naming of disubstituted benzenes follows specific rules based on the relative positions of the substituents, which can be ortho (1,2-), meta (1,3-), or para (1,4-).
Recommended video:
Nomenclature of Aromatic Compounds
The nomenclature of aromatic compounds, particularly disubstituted benzenes, is governed by the IUPAC naming conventions. The base name is derived from benzene, and the substituents are named and numbered according to their positions on the ring. When naming, the substituents are listed in alphabetical order, and the lowest possible numbers are assigned to the substituents to indicate their positions.
Recommended video:
Substituent Effects
Substituent effects refer to how different groups attached to a benzene ring influence its reactivity and the positions where additional substituents can attach. Electron-donating groups (EDGs) tend to direct new substituents to the ortho and para positions, while electron-withdrawing groups (EWGs) typically direct them to the meta position. Understanding these effects is crucial for predicting the behavior of disubstituted benzenes in chemical reactions.
Recommended video:
Naming Other Substituents Example
 Verified step by step guidance
Verified step by step guidance