Textbook Question
Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. a. sodium b. iodine c. calcium d. barium e. krypton
 Verified step by step guidance
Verified step by step guidance

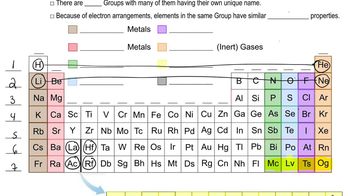

Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. a. sodium b. iodine c. calcium d. barium e. krypton
Classify each element as an alkali metal, alkaline earth metal, halogen, or noble gas. a. F b. Sr c. K d. Ne e. At
Which pair of elements do you expect to be most similar? Why? a. N and Ni b. Mo and Sn c. Na and Mg d. Cl and F e. Si and P
The atomic mass of fluorine is 18.998 amu, and its mass spectrum shows a large peak at this mass. The atomic mass of chlorine is 35.45 amu, yet the mass spectrum of chlorine does not show a peak at this mass. Explain the difference.