Open Question
A sample of Tl-201 has an initial decay rate of 5.88⨉104/s. How long will it take for the decay rate to fall to 287/s? (Tl-201 has a half-life of 3.042 days.)
 Verified step by step guidance
Verified step by step guidance

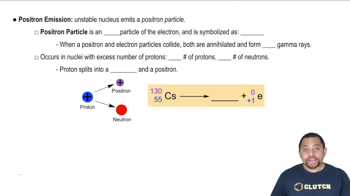

A sample of Tl-201 has an initial decay rate of 5.88⨉104/s. How long will it take for the decay rate to fall to 287/s? (Tl-201 has a half-life of 3.042 days.)
Write a nuclear equation for the indicated decay of each nuclide. a. U-234 (alpha) b. Th-230 (alpha)
Write a nuclear equation for the indicated decay of each nuclide. c. Pb-214 (beta)
Write a nuclear equation for the indicated decay of each nuclide. e. Cr-51 (electron capture)
Write a nuclear equation for the indicated decay of each nuclide. a. Po-210 (alpha) b. Ac-227 (beta)
Write a nuclear equation for the indicated decay of each nuclide.
c. Tl-207 (beta)