Here are the essential concepts you must grasp in order to answer the question correctly.
Radioactive Decay
Radioactive decay is the process by which unstable atomic nuclei lose energy by emitting radiation. This decay occurs at a characteristic rate for each isotope, often described by its decay constant, which is related to the half-life. Understanding this concept is crucial for calculating how the decay rate changes over time.
Recommended video:
Rate of Radioactive Decay
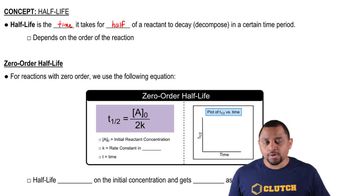
Half-Life
Half-life is the time required for half of the radioactive nuclei in a sample to decay. For F-18, the half-life is 1.83 hours, meaning that after this time, half of the original amount of F-18 will have decayed. This concept is essential for determining the time it takes for the decay rate to decrease from an initial value to a specified lower value.
Recommended video:
Exponential Decay Formula
The exponential decay formula describes how the quantity of a radioactive substance decreases over time. It is expressed as N(t) = N0 * e^(-λt), where N(t) is the quantity at time t, N0 is the initial quantity, λ is the decay constant, and e is the base of the natural logarithm. This formula is fundamental for calculating the time required for the decay rate to reach a specific value.
Recommended video:
 Verified step by step guidance
Verified step by step guidance