Here are the essential concepts you must grasp in order to answer the question correctly.
Titration Curves
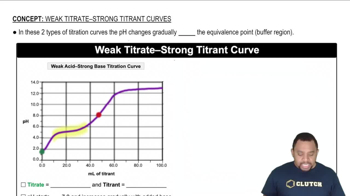
Titration curves graphically represent the change in pH of a solution as a titrant is added. The shape of the curve depends on the strength of the acid and base involved. Strong acids, like HCl, show a sharp increase in pH at the equivalence point, while weak acids, like HF, exhibit a more gradual change due to their incomplete dissociation.
Recommended video:
Acid-Base Titration Curves
Strong vs. Weak Acids
Strong acids, such as HCl, completely dissociate in water, resulting in a high concentration of hydrogen ions (H+). In contrast, weak acids like HF only partially dissociate, leading to a lower concentration of H+. This difference affects the pH at various points in the titration and the overall shape of the titration curve.
Recommended video:
Weak Acid-Strong Base Titration Curve
Equivalence Point
The equivalence point in a titration is reached when the amount of titrant added is stoichiometrically equivalent to the amount of substance in the sample. At this point, the pH of the solution changes dramatically, especially in strong acid-strong base titrations. For weak acids, the pH at the equivalence point will be higher than 7 due to the formation of the conjugate base.
Recommended video: