Two 20.0-mL samples, one 0.200 M KOH and the other 0.200 M CH3NH2, are titrated with 0.100 M HI. b. Is the pH at the equivalence point for each titration acidic, basic, or neutral?
The graphs labeled (a) and (b) show the titration curves for two equal-volume samples of bases, one weak and one strong. Both titrations were carried out with the same concentration of strong acid.
(ii) Which graph corresponds to the titration of the strong base and which one to the weak base?

Verified Solution
Key Concepts
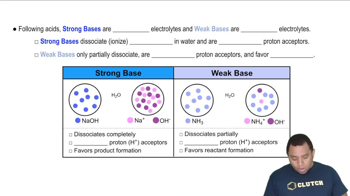
Titration Curves

Strong vs. Weak Bases
Equivalence Point

Two 20.0-mL samples, one 0.200 M KOH and the other 0.200 M CH3NH2, are titrated with 0.100 M HI. c. Which titration curve has the lower initial pH?
Two 20.0-mL samples, one 0.200 M KOH and the other 0.200 M CH3NH2, are titrated with 0.100 M HI. d. Sketch each titration curve.
Consider the curve shown here for the titration of a weak monoprotic acid with a strong base and answer each question.
c. At what volume of added base does pH = pKa?
Consider the curve shown here for the titration of a weak monoprotic acid with a strong base and answer each question.
d. At what volume of added base is the pH calculated by working an equilibrium problem based on the concentration and Kb of the conjugate base?
Consider the curve shown here for the titration of a weak base with a strong acid and answer each question.
a. What is the pH and what is the volume of added acid at the equivalence point?
