Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. a solution that is 0.195 M in HC2H3O2 and 0.125 M in KC2H3O2 b. a solution that is 0.255 M in CH3NH2 and 0.135 M in CH3NH3Br
Ch.17 - Aqueous Ionic Equilibrium
Chapter 17, Problem 33a
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. 0.15 M HF
 Verified step by step guidance
Verified step by step guidance1
Write the balanced chemical equation for the dissociation of HF in water: \( \text{HF} \rightleftharpoons \text{H}^+ + \text{F}^- \).
Set up the ICE table (Initial, Change, Equilibrium) for the concentrations of HF, H⁺, and F⁻. Initially, [HF] = 0.15 M, [H⁺] = 0, and [F⁻] = 0.
Define the change in concentration for the reaction: let \( x \) be the amount of HF that dissociates. At equilibrium, [HF] = 0.15 - x, [H⁺] = x, and [F⁻] = x.
Write the expression for the acid dissociation constant \( K_a \) for HF: \( K_a = \frac{[\text{H}^+][\text{F}^-]}{[\text{HF}]} \). Substitute the equilibrium concentrations into this expression.
Solve the equation for \( x \) to find the concentration of \( \text{H}^+ \), then calculate the pH using the formula \( \text{pH} = -\log[\text{H}^+] \).

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Equilibrium and ICE Tables
Equilibrium in chemistry refers to the state where the concentrations of reactants and products remain constant over time. An ICE table (Initial, Change, Equilibrium) is a tool used to organize the concentrations of species involved in a reaction at different stages. It helps in calculating the changes in concentration as the system reaches equilibrium, which is essential for solving equilibrium problems.
Recommended video:
Guided course

ICE Charts and Equilibrium Amount
Weak Acids and pH Calculation
Weak acids, like hydrofluoric acid (HF), do not completely dissociate in solution, leading to an equilibrium between the undissociated acid and its ions. The pH of a weak acid solution can be calculated using the acid dissociation constant (Ka) and the initial concentration of the acid. The formula pH = -log[H⁺] is used, where [H⁺] can be derived from the equilibrium concentrations established in the ICE table.
Recommended video:
Guided course
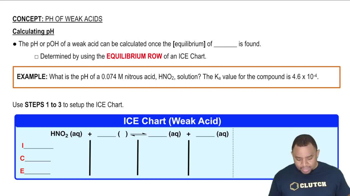
Calculating pH of Weak Acids Example
Acid Dissociation Constant (Ka)
The acid dissociation constant (Ka) quantifies the strength of a weak acid in solution, representing the equilibrium constant for its dissociation into ions. A higher Ka value indicates a stronger weak acid, while a lower Ka suggests a weaker acid. For HF, knowing its Ka value allows for the calculation of the concentration of hydrogen ions at equilibrium, which is crucial for determining the pH of the solution.
Recommended video:
Guided course

Characteristics of Ka and Kb
Related Practice
Textbook Question
Textbook Question
Calculate the percent ionization of a 0.15 M benzoic acid solution in pure water and in a solution containing 0.10 M sodium benzoate. Why does the percent ionization differ significantly in the two solutions?
1978
views
Open Question
What is the percent ionization of a 0.13 M formic acid solution in pure water, and how does it compare to the percent ionization in a solution containing 0.11 M potassium formate?
Textbook Question
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. b. 0.15 M NaF
508
views
Textbook Question
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. c. a mixture that is 0.15 M in HF and 0.15 M in NaF
1765
views
Textbook Question
Solve an equilibrium problem (using an ICE table) to calculate the pH of each solution. a. 0.18 M CH3NH2 b. 0.18 M CH3NH3Cl c. a mixture that is 0.18 M in CH3NH2 and 0.18 M in CH3NH3Cl
