Here are the essential concepts you must grasp in order to answer the question correctly.
Molar Solubility
Molar solubility refers to the maximum amount of a solute that can dissolve in a given volume of solvent at a specific temperature, expressed in moles per liter (mol/L). For calcium hydroxide (Ca(OH)2), which is a sparingly soluble salt, its molar solubility can be influenced by the pH of the solution, as it dissociates into calcium ions (Ca²⁺) and hydroxide ions (OH⁻).
Recommended video:
pH and Hydroxide Ion Concentration
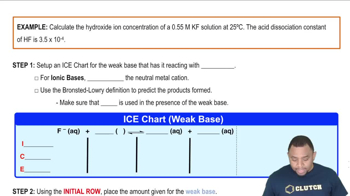
pH is a measure of the acidity or basicity of a solution, with lower values indicating higher acidity. In a buffered solution at pH 4, the concentration of hydrogen ions (H⁺) is high, which can affect the solubility of calcium hydroxide by shifting the equilibrium of its dissociation. The relationship between pH and hydroxide ion concentration (OH⁻) is crucial, as higher H⁺ concentrations can suppress the formation of OH⁻ ions, thereby influencing solubility.
Recommended video:
Hydroxide Ion Concentration Example
Le Chatelier's Principle
Le Chatelier's Principle states that if a dynamic equilibrium is disturbed by changing the conditions, the system will adjust to counteract the change and restore a new equilibrium. In the context of calcium hydroxide solubility, adding H⁺ ions (by lowering pH) shifts the equilibrium towards the solid form of Ca(OH)2, reducing its solubility. Understanding this principle is essential for predicting how changes in pH will affect the molar solubility of the compound.
Recommended video:
 Verified step by step guidance
Verified step by step guidance