Here are the essential concepts you must grasp in order to answer the question correctly.
Buffer Solutions
Buffer solutions are mixtures that resist changes in pH when small amounts of acid or base are added. They typically consist of a weak acid and its conjugate base, or a weak base and its conjugate acid. In this case, the buffer is made up of hypochlorous acid (HClO) and sodium hypochlorite (NaClO), which work together to maintain the pH of the solution.
Recommended video:
Henderson-Hasselbalch Equation
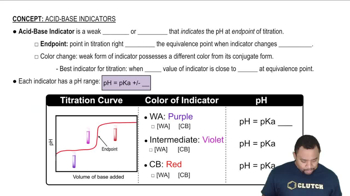
The Henderson-Hasselbalch equation is a mathematical formula used to calculate the pH of a buffer solution. It is expressed as pH = pKa + log([A-]/[HA]), where [A-] is the concentration of the conjugate base and [HA] is the concentration of the weak acid. This equation is essential for determining the pH of the given buffer solution containing HClO and NaClO.
Recommended video:
Henderson-Hasselbalch Equation
pKa and Acid-Base Equilibria
pKa is the negative logarithm of the acid dissociation constant (Ka) and indicates the strength of an acid in solution. A lower pKa value signifies a stronger acid. Understanding the pKa of HClO is crucial for applying the Henderson-Hasselbalch equation to find the initial pH of the buffer solution, as it reflects the equilibrium between the acid and its conjugate base.
Recommended video: