Here are the essential concepts you must grasp in order to answer the question correctly.
Ionization of Acids
Ionization of acids refers to the process by which an acid donates protons (H⁺ ions) to water, resulting in the formation of hydronium ions (H₃O⁺) and the corresponding anions. For polyprotic acids like phosphoric acid (H₃PO₄), this process occurs in multiple steps, each involving the release of one proton at a time.
Recommended video:
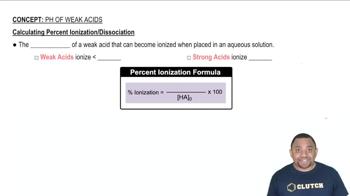
Calculating Percent Ionization of Weak Acids
Equilibrium Expressions
Equilibrium expressions are mathematical representations of the relationship between the concentrations of reactants and products at equilibrium for a given chemical reaction. For ionization reactions, the equilibrium constant (K) is defined as the ratio of the concentration of products to the concentration of reactants, each raised to the power of their coefficients in the balanced equation.
Recommended video:
Equilibrium Constant Expressions
Phosphoric Acid Ionization Steps
Phosphoric acid undergoes three ionization steps, each producing a different anion: H₃PO₄ → H₂PO₄⁻ + H⁺, H₂PO₄⁻ → HPO₄²⁻ + H⁺, and HPO₄²⁻ → PO₄³⁻ + H⁺. Each step has its own equilibrium expression, reflecting the concentrations of the species involved at equilibrium, which are essential for understanding the acid's behavior in solution.
Recommended video:
Calculating Percent Ionization of Weak Acids
 Verified step by step guidance
Verified step by step guidance