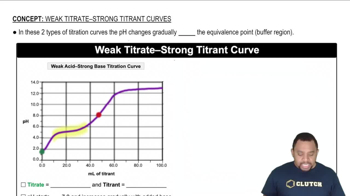
Here are the essential concepts you must grasp in order to answer the question correctly.
Strong vs. Weak Acids
Strong acids, like HCl, completely dissociate in water, producing a high concentration of hydronium ions (H3O+). In contrast, weak acids, such as HF and HClO, only partially dissociate, resulting in a lower concentration of H3O+. Understanding this distinction is crucial for ranking the solutions based on their acidity.
Recommended video:
Weak Acid-Strong Base Titration Curve
Concentration of Solutions
The concentration of an acid solution, expressed in molarity (M), indicates the amount of acid present in a given volume of solution. In this question, all solutions have the same concentration of 0.10 M, but their ability to produce H3O+ varies based on their strength as acids, affecting the final ranking.
Recommended video:
Acid Dissociation Constant (Ka)
The acid dissociation constant (Ka) quantifies the strength of a weak acid in solution. A higher Ka value indicates a stronger acid that dissociates more in water, leading to a greater concentration of H3O+. For the weak acids in the question, knowing their Ka values helps determine their relative acidity and thus their ranking based on H3O+ concentration.
Recommended video:
Characteristics of Ka and Kb