Here are the essential concepts you must grasp in order to answer the question correctly.
Strong Acids and Ionization
Strong acids, like sulfuric acid (H2SO4), completely dissociate in water, meaning that all the acid molecules break apart into ions. For H2SO4, the first dissociation step produces H3O+ and HSO4-, leading to a direct relationship between the concentration of the acid and the concentration of H3O+. Understanding this complete ionization is crucial for calculating pH.
Recommended video:
Strong Acid-Strong Base Titration
pH Calculation
pH is a measure of the acidity of a solution, defined as the negative logarithm of the hydronium ion concentration: pH = -log[H3O+]. For strong acids, the pH can be directly calculated from the concentration of the acid, as the concentration of H3O+ will equal the concentration of the acid due to complete dissociation. This relationship simplifies the calculation of pH for strong acids.
Recommended video:
x is Small Approximation
The 'x is small' approximation is used in equilibrium calculations when the change in concentration (x) is negligible compared to the initial concentration. This approximation is valid when the initial concentration of the acid is significantly higher than the amount that dissociates. For strong acids like H2SO4, this approximation breaks down at higher concentrations, typically when the concentration approaches 0.1 M or higher, where the assumption of negligible change becomes invalid.
Recommended video:
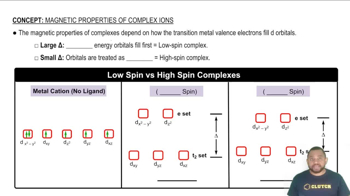
Low-Spin Complexes are associated with large Δ values and High-Spin Complexes are associated with small Δ values.
 Verified step by step guidance
Verified step by step guidance