Consider this energy diagram:
a. How many elementary steps are involved in this reaction?
 Verified step by step guidance
Verified step by step guidance
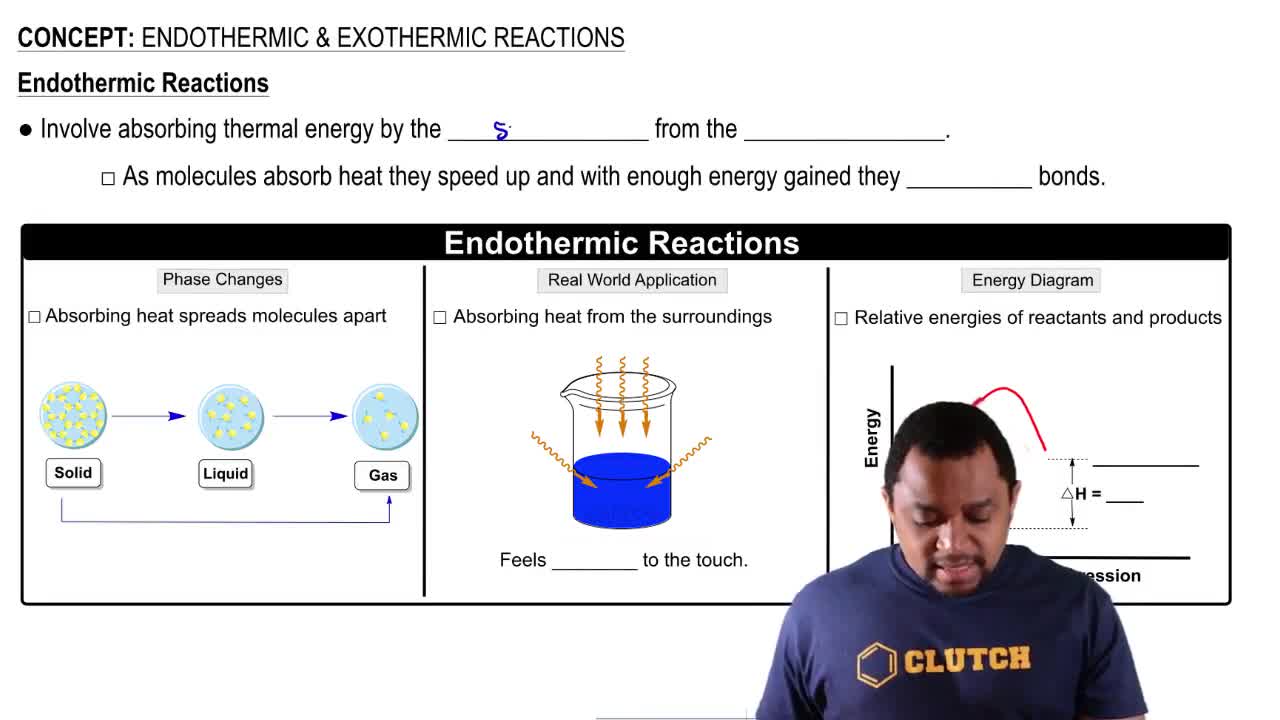


Consider this energy diagram:
a. How many elementary steps are involved in this reaction?
Consider this energy diagram:
<IMAGE>
b. Label the reactants, products, and intermediates.
Consider this energy diagram:
<IMAGE>
c. Which step is rate limiting?
Consider the reaction in which HCl adds across the double bond of ethene: HCl + H2C=CH2 → H3C-CH2Cl The following mechanism, with the accompanying energy diagram, has been suggested for this reaction:
Step 1 HCl + H2C=CH2 → H3C=CH2+ + Cl-
Step 2 H3C=CH2+ + Cl- → H3C-CH2Cl
a. Based on the energy diagram, determine which step is rate limiting.
Consider the reaction in which HCl adds across the double bond of ethene: HCl + H2C=CH2 → H3C-CH2Cl The following mechanism, with the accompanying energy diagram, has been suggested for this reaction:
Step 1 HCl + H2C=CH2 → H3C=CH2+ + Cl-
Step 2 H3C=CH2+ + Cl- → H3C-CH2Cl
b. What is the expected order of the reaction based on the proposed mechanism?