Consider the reaction: NO2(g) → NO(g) + 1/2 O2( g) The tabulated data were collected for the concentration of NO2 as a function of time: b. What is the rate of formation of O2 between 50 and 60 s?
Ch.14 - Chemical Kinetics
Chapter 14, Problem 33aiii
Consider the reaction: H2(g) + Br2(g) → 2 HBr(g) The graph shows the concentration of Br2 as a function of time. a. Use the graph to calculate each quantity: (iii) the instantaneous rate of formation of HBr at 50 s

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Reaction Rate
The reaction rate is a measure of how quickly reactants are converted into products in a chemical reaction. It can be expressed as the change in concentration of a reactant or product over time. Understanding reaction rates is crucial for analyzing how fast a reaction occurs and can be determined from concentration vs. time graphs.
Recommended video:
Guided course

Average Rate of Reaction
Instantaneous Rate
The instantaneous rate of a reaction refers to the rate at a specific moment in time, rather than over an interval. It can be calculated by determining the slope of the tangent line to the concentration vs. time graph at that specific point. This concept is essential for accurately assessing how the concentration of products or reactants changes at a given time.
Recommended video:
Guided course
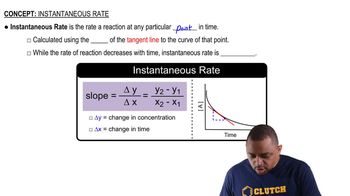
Instantaneous Rate
Stoichiometry
Stoichiometry involves the quantitative relationships between the amounts of reactants and products in a chemical reaction, based on the balanced chemical equation. In the given reaction, the stoichiometric coefficients indicate that for every mole of Br<sub>2</sub> consumed, two moles of HBr are produced. This relationship is vital for converting rates of one substance to rates of another in the reaction.
Recommended video:
Guided course

Stoichiometry Concept
Related Practice
Textbook Question
727
views
Open Question
Consider the reaction: H2(g) + Br2(g) → 2 HBr(g). The graph shows the concentration of Br2 as a function of time. a. Use the graph to calculate each quantity: (ii) the instantaneous rate of the reaction at 25 s.
Textbook Question
Consider the reaction: H2(g) + Br2(g) → 2 HBr(g) The graph shows the concentration of Br2 as a function of time.
a. Use the graph to calculate each quantity: (i) the average rate of the reaction between 0 and 25 s
1368
views
Textbook Question
Consider the reaction: H2( g) + Br2( g) → 2 HBr( g) The graph shows the concentration of Br2 as a function of time.
b. Make a rough sketch of a curve representing the concentration of HBr as a function of time. Assume that the initial concentration of HBr is zero
750
views
Open Question
Consider the reaction: 2 H2O2(aq) → 2 H2O(l) + O2(g). The graph shows the concentration of H2O2 as a function of time. Use the graph to calculate each quantity: a. the average rate of the reaction between 10 and 20 seconds, b. the instantaneous rate of the reaction at 30 seconds.
Textbook Question
Consider the reaction: 2 H2O2(aq) → 2 H2O(l ) + O2( g) The graph shows the concentration of H2O2 as a function of time.
Use the graph to calculate each quantity: c. the instantaneous rate of formation of O2 at 50 s
1892
views
