Textbook Question
An aqueous solution contains 36% HCl by mass. Calculate the molality and mole fraction of the solution.
1601
views


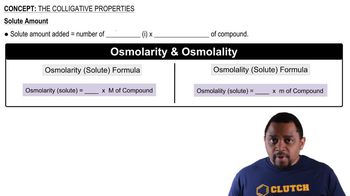

An aqueous solution contains 36% HCl by mass. Calculate the molality and mole fraction of the solution.
Calculate the vapor pressure of a solution containing 24.5 g of glycerin (C3H8O3) in 135 mL of water at 30.0 °C. The vapor pressure of pure water at this temperature is 31.8 torr. Assume that glycerin is not volatile and dissolves molecularly (i.e., it is not ionic), and use a density of 1.00 g/mL for the water.
A solution contains 50.0 g of heptane (C7H16) and 50.0 g of octane (C8H18) at 25 °C. The vapor pressures of pure heptane and pure octane at 25 °C are 45.8 torr and 10.9 torr, respectively. Assuming ideal behavior, answer the following: d. Why is the composition of the vapor different from the composition of the solution?