At high temperatures, sulfur vapor is predominantly in the form of S2(g) molecules. (a) Assuming that the molecular orbitals for third-row diatomic molecules are analogous to those for second-row molecules, construct an MO diagram for the valence orbitals of S2(g).
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
All textbooks McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 107b
Problem 107b
 McMurry 8th Edition
McMurry 8th Edition Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure
Ch.8 - Covalent Compounds: Bonding Theories and Molecular Structure Problem 107b
Problem 107bChapter 8, Problem 107b
Carbon monoxide is produced by incomplete combustion of fossil fuels. (b) Do you expect CO to be paramagnetic or diamagnetic?

Verified Solution
Video duration:
3mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Paramagnetism and Diamagnetism
Paramagnetism occurs in substances that have unpaired electrons, which create a net magnetic moment, allowing them to be attracted to magnetic fields. In contrast, diamagnetism is exhibited by substances with all paired electrons, resulting in no net magnetic moment, causing them to be weakly repelled by magnetic fields. Understanding these properties is essential for predicting the magnetic behavior of molecules.
Recommended video:
Guided course
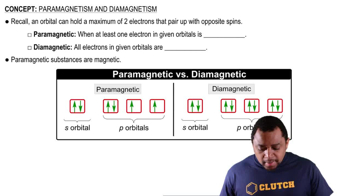
Paramagnetism and Diamagnetism
Molecular Orbital Theory
Molecular Orbital Theory explains how atomic orbitals combine to form molecular orbitals, which can be occupied by electrons. In this theory, electrons are distributed among bonding, antibonding, and non-bonding orbitals. The arrangement of electrons in these orbitals determines whether a molecule is paramagnetic or diamagnetic, based on the presence of unpaired electrons.
Recommended video:
Guided course

Molecular Orbital Theory
Electron Configuration of Carbon Monoxide (CO)
Carbon monoxide (CO) consists of one carbon atom and one oxygen atom, and its electron configuration can be analyzed using molecular orbital theory. In CO, the molecular orbitals are filled according to the Aufbau principle, leading to a configuration where there is one unpaired electron in a π* antibonding orbital. This unpaired electron is what makes CO paramagnetic, as it contributes to a net magnetic moment.
Recommended video:
Guided course

Electron Configuration Example
Related Practice
Textbook Question
466
views
Textbook Question
At high temperatures, sulfur vapor is predominantly in the form of S2(g) molecules. (d) When two electrons are added to S2, the disulfide ion S22- is formed. Is the bond length in S22- likely to be shorter or longer than the bond length in S2? Explain.
513
views
Textbook Question
Carbon monoxide is produced by incomplete combustion of fossil fuels. (a) Give the electron configuration for the valence molecular orbitals of CO. The orbitals have the same energy order as those of the N2 molecule.
345
views
Textbook Question
Carbon monoxide is produced by incomplete combustion of fossil fuels. (c) What is the bond order of CO? Does this match the bond order predicted by the electron-dot structure?
607
views
Textbook Question
Make a sketch showing the location and geometry of the
p orbitals in the nitrite ion, NO2-. Describe the bonding in
this ion using a localized valence bond model for s bonding
and a delocalized MO model for p bonding.
525
views
Textbook Question
In the cyanate ion, OCN-, carbon is the central atom.
(d) Which hybrid orbitals are used by the C atom, and how
many p bonds does the C atom form?
1004
views