Here are the essential concepts you must grasp in order to answer the question correctly.
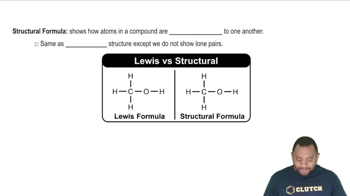
Structural Formula
A structural formula represents the arrangement of atoms within a molecule, showing how the atoms are bonded to each other. It provides insight into the molecular geometry and functional groups present, which are crucial for understanding the chemical properties of the substance. In the case of isopropyl alcohol, the structural formula reveals the presence of a hydroxyl group (-OH) attached to a carbon chain.
Recommended video:
Chemical Formula
The chemical formula of a compound indicates the types and numbers of atoms present in a molecule. For isopropyl alcohol, the chemical formula is C3H8O, which signifies that it contains three carbon atoms, eight hydrogen atoms, and one oxygen atom. This formula is essential for identifying the compound and understanding its chemical behavior.
Recommended video:
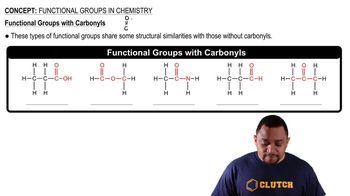
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. In isopropyl alcohol, the hydroxyl group (-OH) is the functional group that classifies it as an alcohol. Understanding functional groups is vital for predicting the reactivity and properties of organic compounds.
Recommended video:
Carbonyl Functional Groups