Write chemical equations for the reaction of potassium with the following substances, making sure that the numbers and kinds of atoms are the same on both sides of the equations. If no reaction occurs, write N.R.
(a) H2O

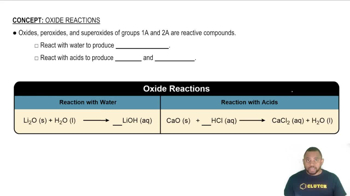


Write chemical equations for the reaction of potassium with the following substances, making sure that the numbers and kinds of atoms are the same on both sides of the equations. If no reaction occurs, write N.R.
(a) H2O
Write chemical equations for the reaction of calcium with the following substances, making sure that the numbers and kinds of atoms are the same on both sides of the equations. If no reaction occurs, write N.R.
(c) Br2
Write chemical equations for the reaction of calcium with the following substances, making sure that the numbers and kinds of atoms are the same on both sides of the equations. If no reaction occurs, write N.R.
(d) O2
Identify the group 3A element that best fits each of the following descriptions.
(a) Is the most abundant element of the group
Identify the group 3A element that best fits each of the following descriptions.
(b) Is stable in the +1 oxidation state
Identify the group 3A element that best fits each of the following descriptions.
(c) Is a semiconductor