Explain why [CoCl4]2- (blue) and [Co(H2O)6]2+ (pink) have different colors. Which complex has its absorption bands at longer wavelengths?
 McMurry 8th Edition
McMurry 8th Edition Ch.21 - Transition Elements and Coordination Chemistry
Ch.21 - Transition Elements and Coordination Chemistry Problem 21.122
Problem 21.122Predict the crystal field energy-level diagram for a square pyramidal ML5 complex that has two ligands along the axes but only one ligand along the z axis. Your diagram should be intermediate between those for an octahedral ML6 complex and a square planar ML4 complex.

Verified Solution
Key Concepts
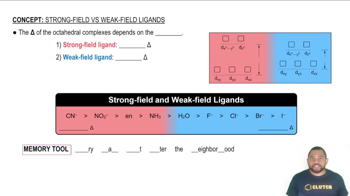
Crystal Field Theory

Ligand Field Strength
Geometry of Coordination Complexes

Look at the colors of the isomeric complexes in Figure 21.12, and predict which is the stronger field ligand, nitro (-NO2) of nitrito (-ONO). Explain.
Predict the crystal field energy-level diagram for a linear ML2 complex that has two ligands along the :
Give a valence bond description of the bonding in each of the following complexes. Include orbital diagrams for the free metal ion and the metal ion in the complex. Indicate which hybrid orbitals the metal ion uses for bonding, and specify the number of unpaired electrons.
(b) [NiBr4]2- (tetrahedral)
For each of the following complexes, describe the bonding using valence bond theory. Include orbital diagrams for the free metal ion and the metal ion in the complex. Indicate which hybrid orbitals the metal ion uses for bonding, and specify the number of unpaired electrons.
(a) [AuCl4]2 (square planar)
For each of the following complexes, describe the bonding using valence bond theory. Include orbital diagrams for the free metal ion and the metal ion in the complex. Indicate which hybrid orbitals the metal ion uses for bonding, and specify the number of unpaired electrons.
(b) [Ag(NH3)2]+