Look at the location in the periodic table of elements A, B, C, and D. What is the electron configuration of the transition metal in each of the following ions?
(c) C3+
(d) DO42-


Look at the location in the periodic table of elements A, B, C, and D. What is the electron configuration of the transition metal in each of the following ions?
(c) C3+
(d) DO42-
What is the systematic name for each of the following molecules or ions? Include cis or trans prefixes for diastereoisomers. Platinum is Pt(II) in square planar complexes and Pt(IV) in octahedral complexes.
(1)
(2)
(3)
(4)
Consider the following isomers [Cr(NH3)2Cl4]-.
(a) Label the isomers as cis or trans.
(b) Which isomers are identical, and which are different?
(c) Do any of these isomers exist as enantiomers? Explain.
Consider the following ethylenediamine complexes.
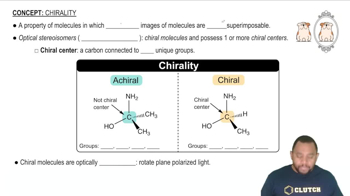
(a) Which complexes are chiral, and which are achiral?
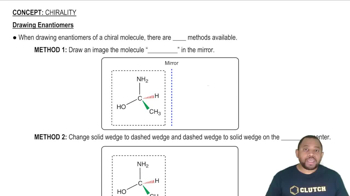
(b) Draw the enantiomer of each chiral complex.
(c) Which, if any, of the chiral complexes are enantiomers of one another?
Consider the following ethylenediamine complexes.
(a) Which complexes are chiral, and which are achiral?
(b) Draw the enantiomer of each chiral complex.
(c) Which, if any, of the chiral complexes are enantiomers of one another?
Use the periodic table to give the electron configuration for each of the following atoms and ions.
(c) Co(V) in CoO43=
(d) Co(IV) in CoF62-