Here are the essential concepts you must grasp in order to answer the question correctly.
Ionic Compounds
Ionic compounds are formed when metals react with nonmetals, resulting in the transfer of electrons. The metal loses electrons to become a positively charged cation, while the nonmetal gains electrons to become a negatively charged anion. The electrostatic attraction between these oppositely charged ions creates a stable compound. Understanding the formation of ionic compounds is essential for writing their chemical formulas.
Recommended video:
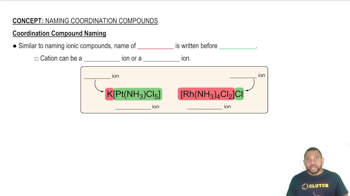
Nomenclature of Coordination Compounds
Coordination compounds consist of a central metal atom bonded to surrounding molecules or ions called ligands. The nomenclature rules for these compounds involve identifying the oxidation state of the metal and naming the ligands appropriately. For example, in Iron(II) cyanide, the Roman numeral indicates the +2 oxidation state of iron, which is crucial for determining the correct formula.
Recommended video:
Coordination Compound Naming
Polyatomic Ions
Polyatomic ions are ions composed of two or more atoms that are covalently bonded and carry a net charge. Common examples include sulfate (SO4^2-) and perchlorate (ClO4^-). Recognizing these ions is vital for accurately writing the formulas of compounds that contain them, as they often dictate the overall charge balance in ionic compounds.
Recommended video:
Polyatomic Ion Variations
 Verified step by step guidance
Verified step by step guidance