Label the following statements about J. J. Thomson's cathode-ray tube experiments shown in Figure 2.6 as true or false. (b) A cathode ray is a stream of charged particles.
Ch.2 - Atoms, Molecules & Ions
Chapter 2, Problem 90
Which of the following charges is not possible for the over-all charge on an oil droplet in Millikan's experiment? For this problem, we'll round the currently accepted charge of an electron to 1.602 * 10-19 C. (a) -1.010 * 10-18 C (b) -8.010 * 10-19 C (c) -2.403 * 10-18 C

Verified Solution
Video duration:
2mWas this helpful?
Key Concepts
Here are the essential concepts you must grasp in order to answer the question correctly.
Elementary Charge
The elementary charge is the smallest unit of electric charge that is considered indivisible, denoted as 'e', with a value of approximately 1.602 x 10^-19 coulombs. In Millikan's oil drop experiment, the charge on the oil droplets is quantized, meaning it can only take on integer multiples of this elementary charge, either positive or negative.
Recommended video:
Guided course

Formal Charge
Quantization of Charge
Quantization of charge refers to the principle that electric charge exists in discrete amounts rather than a continuous range. In the context of Millikan's experiment, this means that the total charge on an oil droplet must be a whole number multiple of the elementary charge, leading to specific allowable charge values for the droplets.
Recommended video:
Guided course

Formal Charge
Millikan's Oil Drop Experiment
Millikan's oil drop experiment was a groundbreaking experiment that measured the charge of the electron by observing the behavior of tiny oil droplets in an electric field. By balancing the gravitational and electric forces acting on the droplets, Millikan was able to determine the charge on the droplets, which confirmed the quantization of charge and provided a precise value for the elementary charge.
Recommended video:
Guided course
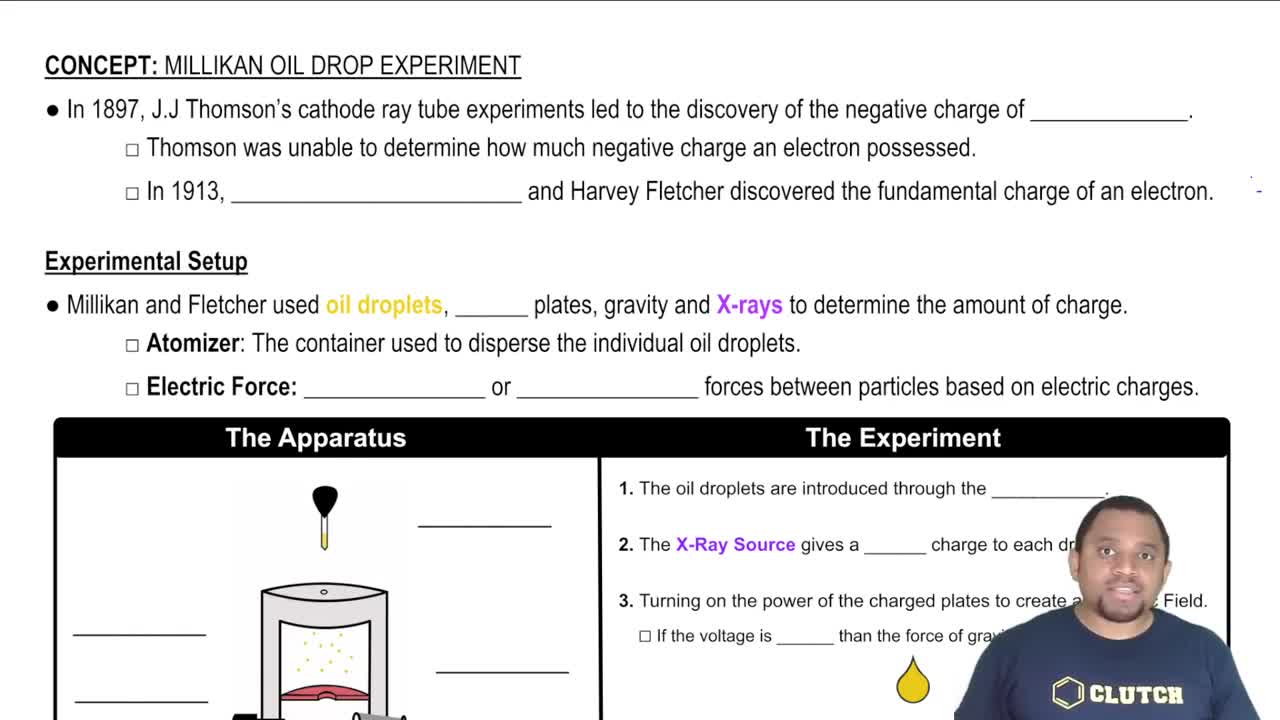
Millikan Oil Drop
Related Practice
Textbook Question
387
views
Textbook Question
Label the following statements about J. J. Thomson's cathode-ray tube experiments shown in Figure 2.6 as true or false. (c) The cathode ray is deflected away from a positively charged plate.
476
views
1
rank
Textbook Question
Label the following statements about J. J. Thomson's cathode-ray tube experiments shown in Figure 2.6 as true or false. (f) By measuring the deflection of the cathode ray beam caused by electric fields of known strength, the charge-to-mass ratio of the electron was calculated.
407
views
Textbook Question
What discovery about atomic structure was made from the results of Rutherford's gold foil experiment?
783
views
Textbook Question
Prior to Rutherford's gold foil experi-ment, the 'plum pudding' model of the atom represented atomic structure. In this model, the atom is composed of elec-trons interspersed within a positive cloud of charge. If this were the correct model of the atom, predict how the results of Rutherford's experiment would have been different.
(a) The alpha particles would pass right through the gold foil with little to no deflection.
(b) Most of the alpha particles would be deflected back toward the source.
(c) Most of the alpha particles would be absorbed by the atom and neither pass through nor be deflected from the gold foil.
586
views
Textbook Question
A period at the end of sentence written with a graphite pencil has a diameter of 1 mm. If the period represented the nucleus, approximately how large is the diameter of the entire atom in units of m?
671
views
