Here are the essential concepts you must grasp in order to answer the question correctly.
Chemical Structure
The chemical structure of a compound refers to the arrangement of atoms within the molecule, including the types of atoms and the bonds between them. Understanding the structure of lactic acid, which includes a hydroxyl group (-OH) and a carboxylic acid group (-COOH), is essential for determining its chemical formula.
Recommended video:
Molecular Formula
A molecular formula represents the number and types of atoms in a molecule. For lactic acid, the molecular formula is derived from its structure, indicating the specific counts of carbon (C), hydrogen (H), and oxygen (O) atoms present in the compound, which is crucial for identifying it correctly.
Recommended video:
Determining Molecular Formulas
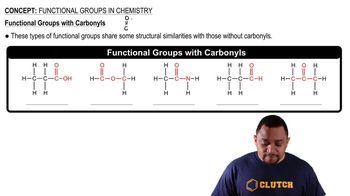
Functional Groups
Functional groups are specific groups of atoms within molecules that are responsible for the characteristic chemical reactions of those molecules. Lactic acid contains both a hydroxyl group and a carboxylic acid group, which influence its properties and reactivity, and are key to understanding its behavior in biological systems.
Recommended video:
Carbonyl Functional Groups