Here are the essential concepts you must grasp in order to answer the question correctly.
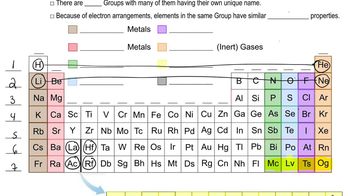
Periodic Table Groups
The periodic table is organized into columns called groups, which categorize elements with similar chemical properties. Groups are numbered from 1 to 18, with specific groups like 1A (alkali metals), 2A (alkaline earth metals), 5A (pnictogens), and 7A (halogens) containing elements that share characteristics. Understanding these groups helps in predicting element behavior and reactivity.
Recommended video:
Periodic Table: Group Names
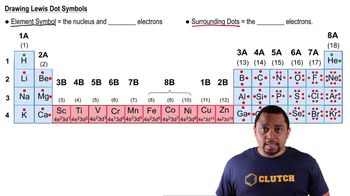
Element Symbols
Each chemical element is represented by a unique one- or two-letter symbol, often derived from its English or Latin name. The first letter of the symbol is always capitalized, while the second, if present, is lowercase. Recognizing the relationship between element names and their symbols is crucial for identifying patterns in the periodic table.
Recommended video:
Nomenclature in Chemistry
Nomenclature refers to the system of naming chemical compounds and elements. In this context, it involves understanding how the names of elements correspond to their symbols. Identifying elements whose symbols start with the same letter as their name requires knowledge of both the elements' names and their respective symbols within the specified groups.
Recommended video: